Increasing Demand of Companion Diagnostics in the Pharmaceutical and Biopharmaceutical Industry
A companion diagnostic assay is an in vitro diagnostic device (IVD) that is used to identify whether a patient with certain diseases could be benefitted by a particular drug through the biomarker assessment. The key factors driving the growth of this market include the increasing lung cancer cases, growing number of genetic testing, rising need for personalized medicines, and regulatory guidelines that support the companion diagnostics market.
What is growth in Companion Diagnostics Market ?
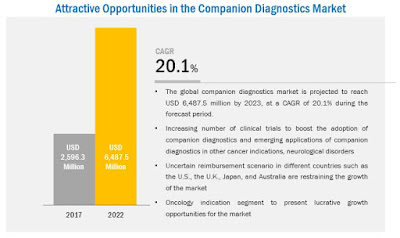
The global companion diagnostics market is expected to reach $6.51 billion by 2022 from $2.17 billion in 2016, at a CAGR of 20.1%. The growth of this market is majorly driven by the factors such as improvements in regulatory guidelines, growing need for targeted therapies, rising cancer incidence across the globe, and increasing collaborations and partnerships for companion diagnostics test development.
Rising adoption of companion diagnostics assay kits & reagents in major applications such as oncology, neurology are driving the growth of companion diagnostics market.
Assay Kits and Reagents
Companion diagnostic assay kits include test panels that enable the detection of biomarker types. Companion diagnostics assays analyze the efficacy of a particular drug through the detection of disease biomarker activity against that drug. Companion diagnostic assays are operated on various technology platforms such as polymerase chain reaction (PCR), next-generation sequencing (NGS), immunohistochemistry (IHC), and in situ hybridization (ISH). Reagents include components such as staining solutions, acids, bases, buffers, detergents, solutions, and substrates required while performing various companion diagnostic tests. Reagents are products paired to the diagnostic test procedures and have a single-use.
Software and Services
Companion diagnostics software is used to streamline the research and analysis of diagnostic results. Software offers various functions such as the identification of driver vs. passenger mutations, prediction of novel biomarkers, and identification of genes that cause cancer. Companion diagnostic services include the development and commercialization of companion diagnostic (CDx) kits, biomarker assay development, analytical and clinical validation, regulatory registration/approval, cGMP manufacturing, GCP/CLIA certified laboratory, and project management. There are many companies offering end-to-end companion diagnostic services from development to commercialization.
Download PDF Brochures – https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=155571681
Which region dominates in Companion Diagnostics Market ?
The companion diagnostic market in APAC is expected to grow at the highest CAGR during the forecast period. Factors such as the growing need of tailored therapeutics for the elderly population; increasing number of hospitals and diagnostic laboratories; and growing prevalence of life-threatening diseases are driving the growth of the companion diagnostics market in this regional segment.
Leading Companies in Companion Diagnostics Market
The prominent players in the global companion diagnostics market are Thermo Fisher Scientific Inc. (U.S.), F. Hoffmann-La Roche AG (Switzerland), Agilent Technologies, Inc. (U.S.), QIAGEN N.V. (Netherland), Illumina Inc. (U.S.).
Critical questions the report answers:
- Where will all these products & services take the industry in the mid to long term?
- What are the current industry developments for companion diagnostics?
View Complete Press Releases –https://www.marketsandmarkets.com/PressReleases/companion-diagnostics.asp

Comments
Post a Comment
If you have any issue, Let me know ?